Infant Total Diet Study (iTDS)
Assessing the exposure of children under 3 years old to certain substances found in food
Following on from the Second Total Diet Study (TDS 2), a national surveillance study of dietary exposure to chemicals, in 2010 ANSES launched the Infant TDS. With this study, the dietary exposure of children under 3 years old was assessed. It confirms the high level of health control demonstrated by the preceding TDSs, but also points out a number of substances requiring special monitoring.
French TDSs
The primary objective of the Total Diet Studies (TDSs), which are carried out on a national scale, is to monitor population exposure to substances of interest in terms of public health. These studies provide data on the estimated composition/contamination of foods "as consumed" and on individual dietary exposure, which is then used to support the implementation of public health policies.
The first EAT, launched by INRA in 2000 in collaboration with AFSSA, produced an overview of the level of exposure of the French population over three years of age to mycotoxins and trace elements and minerals. In 2006, the second TDS was designed to assess dietary exposure of this same population, targeting not only the compounds included in the first TDS but also many substances requiring more detailed knowledge, such as pesticide residues, persistent organic pollutants, additives, phyto-oestrogens and acrylamide. TDS 2 involved the collection of 20,000 food products representing 212 food types, in which 445 substances of interest were screened for.
Finally in 2010, a TDS specifically targeting children under 3 years old was launched.
Why is a specific TDS needed for the infant population?
The infant food market is highly specific and always evolving. Foods intended for infants and young children differ significantly from those for the population groups considered in the previous TDSs. In addition, more knowledge is needed of the infant population’s dietary exposure to chemicals, given its particular vulnerability. The implementation of a total diet study specific to the infant population is therefore essential to provide young children with the best possible protection from the adverse effects of chemicals.
The three stages of the TDS
The TDS consists of three main steps:
- food sampling: purchase of food products and preparation of foods "as consumed" in order to obtain food samples that are representative of individual consumption;
- analysis of samples "as consumed" by accredited laboratories;
- assessment of population exposure and health risks.
A high level of health management, but a few substances to be monitored
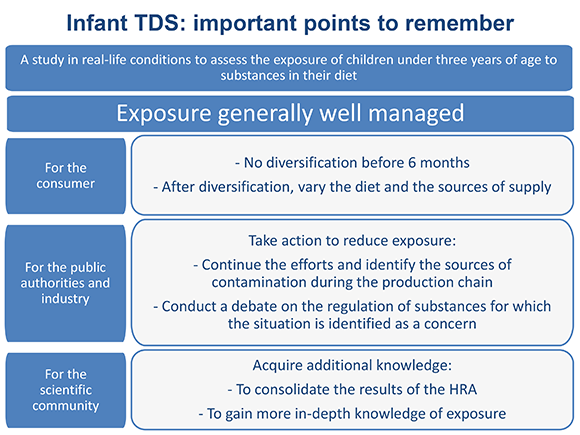
The results of the infant TDS confirm the high level of management of the health risks associated with the potential presence of chemical contaminants in food. In fact, for 90% of the substances assessed, the risk can be ruled out.
However, for nine substances, the situation calls for particular attention. These are substances for which a non-negligible number of children are subject to exposure higher than the toxicity reference values (inorganic arsenic, lead, nickel, PCDD/Fs, PCBs, T-2 & HT-2 mycotoxins, acrylamide, deoxynivalenol and its derivatives, and furan). For seven other substances, in particular aluminium, cobalt, strontium, methylmercury, selenium, cadmium and genistein in soy consumers, the risk cannot be ruled out. Exposure to some of these 16 substances had already been identified as a concern in the Agency's earlier work.
Twelve minerals of nutritional interest were also analysed in the framework of the iTDS. The results show that nutritional needs are generally being met at a satisfactory level. However inadequate intakes for zinc, calcium and iron, and excessive intakes for zinc and calcium were noted, depending on the age of the child. The potential health risks associated with these excessive intakes require additional studies.
The Agency’s recommendations
In light of these findings, ANSES reiterates the importance of better understanding the origin of the presence of these chemicals in food.
Concerning the 16 substances to be monitored, including the nine for which the situation was identified as a concern, management measures aimed at limiting exposure levels should be established or strengthened (policy to control releases into the environment, control of processes, establishment or reduction of regulatory thresholds). Concerning substances for which the risk cannot be excluded or could not be assessed, the Agency recommends acquiring additional knowledge.
As the study also showed that food diversification leads to higher exposure to certain contaminants than that generated by the consumption of infant formulas, without this exposure necessarily being seen as of concern, the Agency stresses the need to follow the recommendations of the National Health and Nutrition Programme (PNNS) and to only begin diversifying the diet from the age of 6 months. After 6 months of age, the Agency reiterates the general recommendation to vary foods and the sources of supply.
The study also highlighted the consumption of normal milk by several children under one year of age. The Agency reiterates that only breast milk or infant formulas can cover an infant's needs. Normal milk, regardless of the animal species that produced it, is not suited to the nutritional needs of children under one year of age.
The iTDS: key figures
- 6 years of work
- More than 200,000 analytical results
- Covering 97% of the diet of children under 3 years of age
- 5,484 products purchased, 457 samples
- 670 substances analysed
- Dietary exposure assessed for 500 substances
- Risk assessed for 400 substances, including 281 pesticide residues